Depo-Provera, a widely used injectable contraceptive, has been linked to serious health risks that are now the focus of growing legal action. While millions of women have relied on Depo-Provera for long-term birth control since its approval in 1992, new scientific studies have uncovered a troubling connection between the drug and the development of meningioma brain tumors.
These findings have sparked a wave of lawsuits across the country, as women who have been diagnosed with meningiomas after using Depo-Provera are now seeking compensation for the harm they’ve suffered. As more evidence emerges, the number of claims against the drug’s manufacturer, Pfizer, is expected to increase.This page will provide the latest news on Depo-Provera lawsuits, explore the legal basis for these claims, and outline what potential plaintiffs can expect regarding compensation and the legal process. If you or a loved one has been injured by Depo-Provera, stay informed about your legal options and the ongoing litigation.
Multidistrict Litigation (MDL) Details
- MDL No. 3140 centralized in the Northern District of Florida before Judge M. Casey Rodgers
- Rapid growth: approximately 130 cases as of April 2025, with over 50 new filings that month
- Plaintiffs allege that long-term use of Depo‑Provera (medroxyprogesterone acetate) caused intracranial meningioma brain tumors, leading to serious neurological harm or the need for surgery
Cases We’re Currently Accepting
- Individuals who received at least two injections of Depo‑Provera or a generic equivalent
- Plaintiffs diagnosed with meningioma brain tumors, especially if it required neurosurgery or resulted in permanent cognitive or neurological damage
Recent Key Litigation Developments
- February 2025: JPML consolidated cases into MDL 3140 under Judge Rodgers in Florida
- April 2025: MDL expands rapidly; over 50 new cases filed in a single month
- June 2025: Parties propose case schedule; preemption motions due September 2025, summary judgment hearings anticipated in late 2025 or early 2026
- State-level litigation also underway in Pennsylvania, California, and Illinois
- Judicial selection process drew attention after a call for diverse leadership; the judge clarified the selection would remain merit-based
The Parties’ Litigation Positions
- Plaintiffs: Argue that Depo‑Provera’s hormonal formulation increases the risk of meningioma and that manufacturers failed to warn the public or adapt product design to mitigate risk
- Defendants: Deny liability, assert that existing FDA-approved labeling was sufficient, and argue that plaintiffs cannot prove a causal link between Depo‑Provera and brain tumors
Settlement Potentials
- No settlements reported as of mid-2025; litigation remains in early discovery stages
- Estimated settlement ranges (if liability is established):
- $275,000–$500,000 for typical meningioma cases
- Higher amounts possible for cases involving aggressive tumors, multiple surgeries, or long-term cognitive impairment
- Actual payouts will depend on tumor severity, impact on quality of life, and proof of use and diagnosis
Major Concerns / Expectations
- Preemption motions scheduled for late 2025 may determine whether many cases are dismissed
- Plaintiffs must provide medical records confirming both Depo‑Provera use and meningioma diagnosis
- Bellwether trials likely in late 2026 or 2027; those outcomes will heavily influence settlement strategy
- Federal MDL growth suggests thousands of potential claims, with parallel state litigation increasing complexity
Depo-Provera Legal Updates and News
As the evidence linking Depo-Provera to serious health risks continues to grow, lawsuits are being filed across the country by women who have developed meningiomas after using the drug. Below are the latest updates on this emerging litigation.
December 1, 2025 - Growth in Federal Filings Plateaus
In the last month, the Depo Provera MDL added just three new cases, bringing the total to 1,225 pending claims. After months of rapid expansion, this slowdown suggests firms may be pacing their filings in batches rather than a steady trickle.
State-court actions continue to progress alongside the federal docket. Recent counts show approximately 332 cases in Delaware, 72 in New York, 21 in California, six in Illinois, and single suits in Pennsylvania, Connecticut, and New Mexico. These parallel filings underscore the nationwide scope of the litigation.
Upcoming Legal Calendar
Pfizer’s motion for summary judgment—arguing that federal law preempts the plaintiffs’ failure-to-warn claims because the FDA rejected its proposed Depo Provera label update—remains pending until the FDA issues its decision on that label change.
Another MDL status conference was scheduled for the end of November; any new orders or deadlines emerging from that meeting will be reported as soon as they become available.
Despite October’s lull, many expect fresh waves of filings in the coming weeks. Women diagnosed with meningioma after Depo Provera use should speak with counsel promptly to protect their rights and meet any looming deadlines.
November 1, 2025 - Federal and State Filings Surge
The Depo-Provera litigation continues its rapid expansion, with 1,346 cases pending in the federal MDL as of October 20, 2025. State-court actions now exceed 439 filings, including Delaware (332), New York (78), California (20), Illinois (6), Pennsylvania (1), Connecticut (1), and New Mexico (1).
The court has set expert disclosures and depositions on general causation for late 2025 and early 2026, when both sides will present scientific testimony on the alleged link between Depo-Provera injections and meningioma brain tumors.
New Case Management Order Issued
Case Management Order No. 6, entered October 17, outlines recent developments in both federal and state forums. Notably, 71 cases have now been filed in New York state court and 19 in California, supplementing the MDL’s growth.
Cohort Study Reinforces Plaintiffs’ Claims
A new population-based cohort study titled Depot Medroxyprogesterone Acetate and Risk of Meningioma in the US found that women using Depo-Provera faced a statistically significantly higher risk of developing meningioma compared with users of oral medroxyprogesterone acetate, other contraceptives, and non-users. Pfizer, however, continues to dispute any causal link in MDL filings. Plaintiffs’ attorneys say this study bolsters their arguments and are citing it in pending motions.
Individual Lawsuit Spotlight
On October 1, Robin Phillip of Louisiana filed suit against Pfizer after developing an intracranial meningioma following years of Depo-Provera injections. Phillip’s complaint alleges neurological damage, vision loss, and insufficient warning about the drug’s tumor risk. Her case underscores the human impact driving this mass-tort surge.
What It Means for Claimants
With federal and state actions mounting, women who used Depo-Provera and later received meningioma diagnoses face critical deadlines. The MDL’s discovery schedule will shape the litigation’s trajectory. Any woman diagnosed with an intracranial meningioma after Depo-Provera use should consult experienced counsel promptly to understand filing windows and evidence requirements.
October 1, 2025 - Strong Pushback Against Preemption Argument and New Study Bolsters Plaintiffs
The Depo-Provera brain tumor litigation has entered a pivotal stage, as the number of cases rises and both sides prepare for a major legal showdown over federal preemption. New scientific research and strategic filings by plaintiffs may significantly shift momentum in the months ahead.
Preemption Motion Filed by Pfizer
Pfizer’s September 2 motion claims that it submitted new safety data to the FDA in 2024 and proposed a warning update. After a nine-month review, the FDA declined to approve the label change. Now, the company says that federal law prevents plaintiffs from suing under state failure-to-warn laws.
However, plaintiffs counter that Pfizer’s request was a last-minute, weak attempt and that the company sat on compelling data for decades. This motion will be critical in determining whether most cases can proceed or get dismissed before full discovery.
Plaintiffs Fight Back on Preemption Argument
On September 22, 2025, plaintiffs filed a strong response to Pfizer’s motion to dismiss the MDL lawsuits based on federal preemption. The company claims it was blocked from adding a warning label about meningioma risks after a 2024 FDA rejection. But plaintiffs say the story is more complicated. They allege:
- Incomplete FDA Communications: Pfizer failed to provide the FDA with complete scientific evidence, including decades of research.
- Mischaracterization of Rejection: The FDA’s 2024 response was not a final denial, and plaintiffs argue it only rejected vague, non-specific language—not a real, risk-based warning.
- Safer Alternatives Ignored: Pfizer decided to remove the lower-dose SubQ formulation from the U.S. market, despite evidence that it could have reduced risks.
- CBE Pathway Was Available: Pfizer could have unilaterally updated the warning label using the FDA’s CBE (Changes Being Effected) process and failed to do so.
Major Study from Cleveland Clinic Strengthens Plaintiffs’ Case
A new study published in JAMA Neurology by Cleveland Clinic researchers adds serious weight to the plaintiffs’ cases:
- Scope: The study reviewed data from over 10 million women across 68 health systems.
- Key Findings: Long-term Depo-Provera users (especially those over age 31 or on the drug for more than four years) had a 2.43x higher risk of developing intracranial meningioma.
- No Similar Risk: Other contraceptives studied showed no elevated risk.
- Real-World Impact: For every 1,100 women exposed, one additional brain tumor is expected.
This study may significantly influence future Daubert challenges (on expert testimony) and reduce Pfizer’s ability to argue that U.S.-specific data is lacking.
MDL Case Count Surges to 800+
As of last month, more than 800 lawsuits have been filed in the federal MDL, up from 550 in August. This sharp increase reflects growing awareness and momentum behind the litigation.
Kaiser Named in California Lawsuit
On September 8, a California woman filed a new state lawsuit in Alameda County, naming not only Pfizer but also Kaiser Permanente entities as defendants. The plaintiff claims:
- She received Depo-Provera injections at Kaiser facilities from 2016 to 2024.
- She developed multiple brain tumors, one of which required surgery.
- Kaiser allegedly continued to promote Depo-Provera despite known risks, and falsely portrayed its safety reviews as independent and reliable.
This lawsuit may foreshadow more cases where health care systems are named alongside drug manufacturers.
September 1, 2025 - Preemption Fight Intensifies as Lawsuits Multiply
The multidistrict litigation (MDL) over Pfizer’s injectable contraceptive Depo-Provera continues to grow in scope and legal complexity. As of August 2025, more than 550 lawsuits have been filed in MDL No. 3140 in the Northern District of Florida, with additional cases emerging across multiple state courts.
Preemption Becomes Central Legal Battle
At the core of the litigation is a looming decision on federal preemption, which could dramatically shape the future of the case. Pfizer argues that the FDA’s 2024 rejection of its proposed warning label for Depo-Provera prevents the company from being sued under state failure-to-warn laws. If successful, this could shut down most of the MDL before full discovery begins.
Plaintiffs, however, are expected to argue that Pfizer’s proposed label language was too vague and lacked scientific specificity. They claim the FDA rejected the language, not the warning itself, and that Pfizer could have used its authority to independently strengthen the warning. A hearing on the matter is scheduled for September 29, 2025.
Judge Requests Transparency on Unfiled Claims
Judge M. Casey Rodgers recently ordered plaintiffs’ leadership to disclose the number of unfiled claims in their possession. The court aims to prevent a potential surge in filings following the preemption ruling, which could disrupt case management and settlement negotiations. The move reflects concerns raised in other MDLs, such as AFFF and Zantac, where large numbers of unfiled claims complicated the litigation timeline.
New Jersey Plaintiff Alleges Life-Altering Brain Tumor
A new complaint filed directly in the MDL alleges that a New Jersey woman developed an intracranial meningioma after more than a decade of Depo-Provera use. Diagnosed in 2023, she underwent brain surgery and continues to experience lasting harm. The lawsuit cites Pfizer’s failure to warn U.S. consumers despite international regulators issuing risk disclosures and points to a safer, lower-dose alternative — DepoSubQ Provera 104 — which the company allegedly failed to promote.
Delaware Sees Surge in State Court Filings
State court litigation is also expanding. A new Delaware complaint includes 100 plaintiffs who opted to file outside the federal MDL. Delaware’s status as Pfizer’s state of incorporation may offer strategic advantages to plaintiffs seeking faster or more flexible proceedings. Similar activity is underway in New York (61 cases) and California (11), with other filings in Illinois, Pennsylvania, and New Mexico.
This trend raises the possibility that one of these state cases could proceed to trial before a federal bellwether test trial, potentially setting early expectations for settlement negotiations.
August 1, 2025 - Federal and State Depo-Provera Case Counts Grow
The Depo-Provera multidistrict litigation (MDL) continues to expand, with more than 550 lawsuits now centralized in the Northern District of Florida. At the same time, a modest but growing number of state court filings suggests a parallel front may be developing.
State Courts Show Emerging Activity
While the MDL remains the primary venue for most claims, state-level litigation is gradually picking up. New York leads with 61 active cases, awaiting assignment to a coordination judge. California follows with 11 lawsuits, with a petition for judicial coordination still pending. Additional filings are scattered across Pennsylvania, Illinois, Delaware, and New Mexico.
This wider geographic footprint may increase legal pressure on Pfizer and other named defendants as cases advance on multiple tracks.
Wrongful Death Lawsuit Filed in Texas
A new wrongful death claim was filed in the federal MDL by the family of a Texas woman who died at age 47 from a sudden brain hemorrhage allegedly caused by an undiagnosed intracranial meningioma linked to long-term Depo-Provera use.
The decedent, Anita Petersen, reportedly used the contraceptive injection for years, including during six years in Minnesota, before her death in October 2024. An autopsy determined the cause of death was a tumor-related hemorrhage. The lawsuit joins a growing body of claims alleging that Pfizer failed to adequately warn about Depo-Provera’s brain tumor risks.
Questionnaire Deadlines Strictly Enforced
The MDL judge continues to enforce pretrial deadlines aggressively. Plaintiffs who miss deadlines for submitting their Plaintiff Profile and Proof of Use Questionnaires are now routinely placed under Show Cause orders. This procedural mechanism demands immediate compliance and could jeopardize a claim if left unaddressed.
The burden of documentation is lighter for those participating in the medical monitoring class. To meet the minimum standards for questionnaire sufficiency, these individuals must provide proof of use only, not proof of injury.
If you have any questions about bringing a Depo-Provera lawsuit, the team at Lawsuit Legal News has answers.
July 1, 2025 - Federal and State Cases Move Forward
The Depo-Provera MDL continues to grow, with over 400 cases now pending in federal court and an expanding footprint in state courts nationwide. Here’s where the litigation stands heading into July.
Federal MDL Moves Toward Major Preemption Fight
On June 19, parties in the MDL proposed a revised case schedule that positions preemption briefing, a critical issue in the litigation, for resolution by late September. Discovery related to preemption is set to close July 25, with summary judgment motions due August 22 and oral arguments scheduled for September 29, 2025.
This hearing will address Pfizer’s central defense: that federal law preempts state-level failure-to-warn claims because the FDA previously declined to approve a brain tumor warning for Depo-Provera. Plaintiffs counter that Pfizer had an independent duty to act in light of new safety information, including emerging studies on the drug’s tumor risk.
While this motion could potentially remove hundreds of cases from the MDL, plaintiffs remain confident that the judge will allow the litigation to move forward.
New Lawsuits Filed as MDL Activity Intensifies
A Massachusetts woman filed a new case on June 13, alleging she developed a meningioma after receiving Depo-Provera injections between 2016 and 2022. Her tumor required aggressive radiation treatment and left her with lasting neurological and psychological harm. She joins hundreds of others in the growing MDL, which continues to attract new plaintiffs from across the country.
State Court Efforts Evolve
Momentum in Pennsylvania has stalled after early signs of mass tort coordination. A judge recently severed more than 100 plaintiffs from a consolidated Philadelphia case, and no new filings followed. Pfizer has since withdrawn its petition for coordination, leaving only one case active in that jurisdiction for now.
In contrast, state court proceedings are accelerating elsewhere. New York now leads with 60 active cases, and coordination appears imminent. The court has stayed nearly all cases pending a decision, and both parties have agreed to centralized case management. California, meanwhile, has six pending cases and a coordination petition under review. Additional lawsuits are progressing in Illinois, Delaware, and New Mexico.
Proof of Use and Records Collection Continue
As required by earlier pretrial orders, plaintiffs are actively submitting documentation of Depo-Provera use and brain tumor diagnoses. As of mid-June, 34 completed submissions had been received. Attorneys are working to identify and resolve documentation deficiencies while monitoring for problems with noncompliant medical providers. If access issues persist, plaintiffs may ask the court to compel production under a HIPAA-compliant order.
June 1, 2025 - Depo-Provera Brain Tumor Lawsuits Gain Ground with Stronger Evidence and Procedural Clarity
The Depo-Provera multidistrict litigation (MDL) continues to build momentum as new scientific findings, legal filings, and court orders reinforce plaintiffs’ claims that the birth control injection may significantly increase the risk of brain tumors, specifically intracranial meningiomas.
A Safer Alternative and Missed Opportunity
One of the latest issues in the spotlight is Pfizer’s decision not to actively promote a lower-dose version of the drug, Depo Sub-Q Provera 104, which delivers just 104 mg of medroxyprogesterone acetate (MPA) via subcutaneous injection, compared to the 150 mg delivered intramuscularly in the traditional version.
Many medical experts believe that long-term use of the lower-dose alternative could have meaningfully reduced the risk of meningioma by limiting overall exposure to synthetic progestin. Plaintiffs’ attorneys now argue that Pfizer’s failure to transition patients to the lower-dose product was a business-driven decision, not a medical one. This alleged design defect is expected to become a key argument in the litigation.
New Case Filed by Iowa Plaintiff
On May 16, a woman from Iowa City filed a new lawsuit in the MDL, alleging that her use of Depo-Provera from 2008 to 2016 caused her to develop a debilitating brain tumor. After moving to Iowa, she was diagnosed with a meningioma in 2023 following symptoms like slurred speech, numbness, and double vision. Her case adds to the growing number of long-term users coming forward with serious health complications allegedly linked to the contraceptive injection.
Study Confirms Serious Risk
The scientific foundation of the litigation was strengthened by a recent University of British Columbia study led by Connor Frey and Mahyar Etminan. The researchers found that women who used Depo-Provera for over a year had a 3.55 times higher risk of developing an intracranial meningioma compared to women using an oral contraceptive (EE-LNG).
Their analysis corrected several shortcomings of the earlier Roland study by using a larger sample size, an active comparator, and appropriate lag periods to address disease latency. The study’s results suggest one in every 1,111 women using Depo-Provera for three years may develop a brain tumor, and they bolster claims that the U.S. label failed to warn of this risk, even as European regulators required such warnings.
Projected Case Volume and Strategy
Although the MDL added just two new cases in April—bringing the total to 289—plaintiffs’ attorneys predict a wave of new filings over the summer. With 2–3 million Depo-Provera prescriptions filled annually in the U.S., and research suggesting a 5.5-fold increase in meningioma risk, legal analysts estimate that over 1,000 brain tumor cases per year may be tied to the drug. Historically, only a fraction of eligible patients pursue claims, but if even 5–20% come forward, the number of lawsuits could triple or more in the coming months.
Attorneys are prioritizing the strongest cases first—those involving long-term use, serious diagnoses, and extensive medical documentation. These early cases are likely to become bellwethers, shaping the court’s views on liability and damages, and potentially setting the tone for future settlements.
Court Orders and Record-Keeping Requirements
To keep the MDL organized and efficient, Judge Rodgers issued Pretrial Orders Nos. 22 and 23 in May. These orders lay out strict standards for what plaintiffs must include in their complaints and medical documentation:
Encouragement for Early Compliance: The court has encouraged attorneys to correct any issues proactively rather than wait to be flagged. While the judge acknowledged the challenges in obtaining older medical records, she also emphasized the need for prompt and complete submissions to keep cases moving forward.
Proof of Use and Injury: Plaintiffs must complete a detailed questionnaire and provide documentation showing they used Depo-Provera and were diagnosed with a qualifying meningioma. Submissions must follow specific formatting and length guidelines.
Deficiency Process: BrownGreer, the court’s third-party administrator, will review complaints and notify attorneys of any deficiencies. Plaintiffs have a short window to amend their filings or risk dismissal.
May 1, 2025 - Depo-Provera MDL Sees Rapid Growth and Momentum
The Depo-Provera multidistrict litigation (MDL) is gaining traction, with more women coming forward to file claims alleging serious brain injuries linked to long-term use of the injectable contraceptive. In April 2025 alone, 52 new cases were added to the MDL, nearly doubling its size to 130 total lawsuits. That number is expected to increase quickly in the weeks ahead, especially once the court finalizes a short-form complaint to streamline the filing process.
Many of the plaintiffs allege that extended use of Depo-Provera (also known as DMPA) caused the development of intracranial meningiomas—a type of brain tumor. These claims often involve permanent neurological damage and invasive surgeries. Central allegations include failure to warn about risks, defective drug design, and failure to disclose safer alternatives.
New Lawsuits Filed in Kentucky, Tennessee, and Florida
Several high-profile cases were added in April:
- A Tennessee woman alleges that long-term Depo-Provera use led to multiple brain tumors, including one that required a craniotomy in 2013. She resumed injections later, only to develop a second tumor in 2018.
- A Kentucky plaintiff claims that she used Depo-Provera from 2001 to 2006 and developed a brain tumor more than a decade later, which required surgery and left her with lasting neurological issues.
- A Florida woman is also joining the MDL, stating that she developed sensorineural hearing loss and tinnitus caused by a meningioma linked to Depo-Provera injections from the early 2000s.
In each of these cases, plaintiffs emphasize that international health regulators in countries like Canada, South Africa, and those in the EU have long required warnings about the risk of meningioma — yet U.S. labels remain silent on the issue.
Legal Action Against Generic Manufacturers Moves Forward
The role of authorized generic manufacturers—Greenstone, Viatris, and Prasco—has been under scrutiny. A recent court order clarified where each stands:
- Prasco may soon be dismissed from the MDL, pending final stipulation.
- Greenstone and Viatris, however, remain active defendants. The judge denied any early dismissal and is requiring them to fully participate in discovery.
- While both companies submitted affidavits claiming limited involvement, the court reserved the right to revisit the issue later in the litigation.
For plaintiffs, this means that Greenstone and Viatris will still be subject to discovery obligations, and their potential liability remains under investigation.
Judge Rodgers Emphasizes Strict Compliance
At the latest case management conference, the court sent a strong message about deadlines and accountability. After Greenstone and Viatris failed to timely submit court-ordered affidavits, Judge Rodgers issued an Order to Show Cause, compelling them to comply. Both eventually provided the required documentation, but the judge’s firm stance makes it clear: all parties must stay on schedule or face consequences.
Proof-of-Use Orders Help Plaintiffs Move Forward
One of the key hurdles in this litigation is proving that each plaintiff actually received Depo-Provera or its generic equivalent. To address this, the court has established a uniform process for collecting product use records.
Under this new order:
- Plaintiffs must complete a Proof of Use and Injury Questionnaire within 120 days of March 14, 2025 (or of filing, if added later).
- The order compels third parties—like clinics, pharmacies, military health systems, and insurers—to release records showing whether a plaintiff received DMPA injections, even if medical charts are unavailable.
- Plaintiffs can use court-approved HIPAA-compliant forms and, if needed, serve subpoenas with fewer procedural hurdles.
This order is a critical step that will help many plaintiffs overcome documentation challenges, especially in older cases where records may be missing or incomplete.
Earlier Depo-Provera Litigation News
Once the multidistrict litigation was formed for these cases in February 2025, the judge has kept the pressure on both sides to move these cases to resolution. Important motion filing deadlines have been established and expert witness challenges will be decided by early next year.
At the same time, many injured plaintiffs are filing their claims in state courts instead of following the federal MDL process. Discovery is continuing, and the court appointed a leadership team to represent the hundreds of plaintiffs involved in these cases.
Frequently Asked Questions About the Depo-Provera Lawsuits
What health risks are linked to long-term use of Depo-Provera?
Long-term use of Depo-Provera, an injectable contraceptive, has been associated with serious health risks, most notably the development of intracranial meningioma brain tumors. Scientific studies have found that women who used Depo-Provera for over a year had a significantly higher risk—up to 3.55 times greater—of developing meningiomas compared to those who used oral contraceptives. These tumors, though typically non-cancerous, can cause severe neurological symptoms and often require invasive treatments like surgery or radiation. The drug has also been linked to blood clots in rare cases, raising additional safety concerns.
What is the basis of the lawsuits being filed against Depo-Provera’s manufacturer, Pfizer?
The lawsuits claim that Pfizer, the maker of Depo-Provera, failed to adequately warn users about the increased risk of brain tumors. Plaintiffs allege that the company knew, or should have known, about the dangers of long-term use but did not include sufficient warnings on U.S. product labels. The lawsuits focus on two primary legal theories: failure to warn and defective design. Some claims also argue that Pfizer continued to promote the higher-dose version of Depo-Provera instead of a safer, lower-dose alternative (Depo Sub-Q Provera 104) to preserve market share, putting profits ahead of patient safety.
What types of injuries are women experiencing after using Depo-Provera?
Women filing lawsuits report developing meningioma brain tumors, which can lead to symptoms like chronic headaches, vision problems, slurred speech, memory loss, and cognitive difficulties. In many cases, these tumors required invasive brain surgeries or radiation treatments. Some women continue to suffer from permanent neurological damage, chronic pain, or psychological effects long after their diagnosis and treatment. In the most severe cases, the tumors are inoperable, leading to long-term impairment.
What scientific evidence supports the claims in the Depo-Provera lawsuits?
A recent study from the University of British Columbia found that women who used Depo-Provera for more than one year had a 3.55x higher risk of developing intracranial meningiomas compared to users of other contraceptives. The study used a large sample size and corrected flaws from earlier research by including proper lag periods and an active comparator. Additional evidence shows that Depo-Provera’s synthetic hormone, medroxyprogesterone acetate (MPA), can overstimulate hormone receptors in the brain, increasing the risk of tumor development.
Why is the lower-dose version of Depo-Provera relevant to these lawsuits?
Pfizer also manufactures a lower-dose alternative called Depo Sub-Q Provera 104, which delivers 104 mg of MPA instead of 150 mg. This version is injected subcutaneously and may reduce hormone exposure and associated health risks. Plaintiffs argue that Pfizer failed to promote or transition users to this safer formulation for business reasons. This alleged design defect is expected to play a central role in litigation, as it suggests that Pfizer knowingly continued to market a more dangerous version of the drug.
How many Depo-Provera lawsuits have been filed so far?
As of mid-2025, over 400 Depo-Provera lawsuits have been filed in federal court, with many more emerging in state courts across the U.S. The litigation is now part of a Multidistrict Litigation (MDL) in the Northern District of Florida, where similar claims are consolidated for efficient pretrial handling. This number is expected to grow significantly, as studies estimate that thousands of women may have developed brain tumors linked to Depo-Provera use.
What is the current status of the Depo-Provera MDL?
The MDL is currently in the discovery phase, with summary judgment motions on Pfizer’s preemption defense scheduled for August 2025, and oral arguments set for September 29, 2025. Discovery on general causation will continue into late 2025, with bellwether trials anticipated in 2026. These early trials will serve as test cases and could influence future settlements or verdicts. The litigation is moving swiftly under Judge M. Casey Rodgers, who has experience managing high-profile pharmaceutical cases.
What documentation is required to file a Depo-Provera lawsuit?
Plaintiffs must provide:
- Proof of Depo-Provera use (e.g., prescriptions, pharmacy records)
- Medical records confirming a meningioma diagnosis
- Details about symptoms, treatments, and outcomes
The court has issued strict guidelines for submitting this information. Plaintiffs must also complete a formal questionnaire and respond to any deficiencies identified by the court-appointed administrator. Missing or incomplete documentation can delay or jeopardize a claim.
Why didn’t the FDA warn U.S. consumers about Depo-Provera’s tumor risks?
Although European regulators like the EMA required Depo-Provera labels to include warnings about meningiomas, the U.S. FDA has not mandated similar updates. Pfizer has not voluntarily added these warnings to the U.S. label, which remains silent on this serious risk. Lawsuits allege this omission deprived patients of the chance to make informed decisions about their health, and that Pfizer failed its duty to update safety information as new studies emerged.
What types of compensation are being pursued in Depo-Provera lawsuits?
Plaintiffs are seeking compensation for:
- Medical expenses, including surgery, treatment, and ongoing care
- Lost wages or earning capacity due to health complications
- Pain and suffering, including physical pain and emotional distress
- Punitive damages, in cases where Pfizer is found to have acted recklessly
Some lawsuits also seek damages for long-term disability or permanent impairments resulting from brain tumors and their treatment.
What Are the Legal Grounds for Depo-Provera Lawsuits?
The lawsuits being filed against Pfizer, the manufacturer of Depo-Provera, are primarily based on claims of product liability and failure to warn. These legal actions assert that Pfizer did not provide adequate warnings about the severe risks associated with prolonged use of the drug, particularly the increased risk of developing meningioma brain tumors.
Failure to Warn
Under U.S. product liability law, drug manufacturers must warn consumers and healthcare providers about known risks associated with their products. Plaintiffs in the Depo-Provera lawsuits argue that Pfizer failed to meet this obligation. Although scientific studies have shown a link between Depo-Provera and meningiomas, these risks were not reflected in the drug’s warning labels or patient information leaflets, particularly in the U.S.
In Europe, Pfizer updated Depo-Provera’s warning label to include information about the increased risk of brain tumors. Still, these warnings were not included in the U.S. versions of the drug’s labeling. Plaintiffs claim that had they been adequately informed of the potential dangers, they would have chosen different contraceptive methods or monitored their health more closely for signs of brain tumors.
Product Liability
The claims also focus on strict product liability, which holds manufacturers accountable for distributing unreasonably dangerous products to consumers. Even if Pfizer was not negligent in designing Depo-Provera, plaintiffs allege that the drug was inherently hazardous due to its link to meningiomas. They argue that Pfizer should be held liable for the harm caused by the drug, regardless of intent or negligence.
Negligence
In addition to product liability and failure to warn, some plaintiffs pursue negligence claims. These claims argue that Pfizer failed to test the long-term effects of Depo-Provera properly and failed to disclose the known risks to the public promptly. Legal teams are also investigating whether Pfizer deliberately withheld information about these risks, further strengthening the case for negligence claims.
The Depo Provera Lawsuit is an ACTIVE Lawsuit
Multidistrict Litigation (MDL) and Class Actions
Given the number of lawsuits being filed, the Depo-Provera cases are expected to be consolidated into a multidistrict litigation (MDL). In an MDL, similar cases are grouped in one court to streamline pretrial proceedings, making the process more efficient.
While plaintiffs retain their lawsuits, common legal and factual issues are addressed collectively. This approach often leads to faster settlements, especially if early trials (known as bellwether trials) indicate a strong case for the plaintiffs.
Filing a Depo-Provera lawsuit may seem daunting, but understanding the legal process can help make it more manageable. Below is a step-by-step breakdown of what plaintiffs can expect when pursuing legal action against Pfizer.
1. Initial Consultation and Case Evaluation
The first step in filing a Depo-Provera lawsuit is consulting with an attorney experienced in product liability and pharmaceutical cases. At Lawsuit Legal News, our supporting law firm, Dolman Law Group, offers free case consultations nationwide to help you determine if you are eligible to file a lawsuit for your injuries.
During this consultation, our legal team will evaluate your case by reviewing medical records, your history of Depo-Provera use, and your diagnosis of a brain tumor, such as a meningioma. We will also assess the strength of your claim based on factors like how long you used the drug and whether you were warned of the potential risks. This information will help us decide whether your case would qualify for a Depo-Provera lawsuit under the circumstances of the case.
2. Gathering Medical Documentation
Our legal team must gather all relevant medical records to build a strong case. This includes documentation that shows your use of Depo-Provera, such as prescriptions or billing records from your healthcare provider, as well as records of your diagnosis, treatment, and any surgeries related to a meningioma or other brain tumors. Medical records are essential to proving that the drug caused your injuries.
3. Filing the Lawsuit
Once your case has been evaluated and the necessary documentation gathered, your attorney will file a lawsuit against Pfizer on your behalf. This legal document, known as a complaint, outlines your claims and the damages you seek. The lawsuit may be filed individually or as part of a larger multidistrict litigation (MDL), where similar claims are grouped to streamline the legal process.
4. Discovery Process
After the lawsuit is filed, the discovery phase begins. Both sides exchange evidence during this stage, including medical records, internal company documents, and expert testimony. This phase is crucial for building a solid case, as it may uncover information about what Pfizer knew regarding the risks of Depo-Provera and when they knew it. Expert testimony from medical professionals and scientists will also play a key role in establishing the link between Depo-Provera and brain tumors.
5. Bellwether Trials and Settlement Negotiations
In large pharmaceutical cases like Depo-Provera, early bellwether trials are often held to test the strength of the plaintiff's claims and the evidence. These trials help determine how future cases may proceed and can influence settlement negotiations. If the bellwether trials result in favorable outcomes for plaintiffs, it often leads to settlement discussions where Pfizer may agree to compensate victims without going to trial.
6. Settlement or Trial
Many Depo-Provera cases are expected to settle before reaching trial. Settlements provide victims with compensation without the uncertainty and lengthy trial process. However, if a settlement cannot be reached, the case may go to trial, where a jury will decide whether Pfizer is liable for the harm caused by the drug and determine the amount of compensation.
Throughout this process, your attorney will guide you, handle all legal proceedings, and work to secure the compensation you deserve. Most importantly, by filing a lawsuit, you can help hold pharmaceutical companies accountable for their actions and prevent future harm to others.
What Compensation is Available in Depo-Provera Cases?
For those who have suffered severe health issues after using Depo-Provera, pursuing a lawsuit offers the possibility of financial compensation. The compensation available in Depo-Provera cases varies depending on the extent of the injury, the impact on the individual’s life, and the strength of the legal claim. The following are the types of damages victims may be able to recover through a lawsuit.
Medical Expenses
One of the most significant areas of compensation in Depo-Provera lawsuits is the recovery of medical costs. These expenses may include.
- Diagnosis and Treatment. Costs related to diagnosing brain tumors, including imaging tests like MRIs or CT scans, and treatment such as surgery or radiation therapy to remove or manage meningiomas.
- Ongoing Care. Long-term medical care, such as follow-up appointments, neurological evaluations, and regular monitoring for tumor regrowth.
- Medications. Prescription drugs are used to manage symptoms or prevent the recurrence of the tumor.
- Rehabilitation and Therapy. Costs associated with physical therapy, occupational therapy, or other rehabilitation services necessary after surgery or treatment.
Lost Income
For many victims, the recovery process from a meningioma can involve long periods away from work, either temporarily or permanently. Compensation for lost income covers.
- Wages Lost During Recovery. If a plaintiff cannot work while undergoing surgery or treatment, they may be compensated for their lost income.
- Loss of Earning Capacity. In cases where the plaintiff’s ability to work is permanently diminished due to their health condition, they may be entitled to compensation for future lost income or reduced earning capacity.
Pain and Suffering
Compensation for pain and suffering addresses the physical and emotional toll of dealing with a severe health condition like a brain tumor. This includes.
- Physical Pain. The pain associated with surgery, recovery, and ongoing health problems caused by Depo-Provera.
- Emotional Distress. The anxiety, depression, and psychological trauma that often accompany a brain tumor diagnosis and treatment, including the fear of tumor recurrence.
- Diminished Quality of Life. Many plaintiffs experience a reduced quality of life due to long-term neurological complications, chronic headaches, or cognitive difficulties, which can be compensated through pain and suffering damages.
Punitive Damages
In some cases, courts may award punitive and compensatory damages. Plaintiffs in Depo-Provera lawsuits may argue that Pfizer knew, or should have known, about the risks of meningiomas but failed to warn consumers, warranting punitive damages to hold the company accountable for its actions.
Other Economic Damages
Victims may also be compensated for other economic losses caused by their condition.
- Travel Expenses. Costs associated with traveling to medical appointments or receiving specialized care.
- Home Modifications. If a plaintiff’s condition requires home modifications (e.g., ramps or other accessibility features), they may be compensated for these expenses.
How will the settlement amounts in the Depo-Provera lawsuit be determined and how much will they be?
Which Factors Influence Compensation?
The compensation a plaintiff may receive in a Depo-Provera lawsuit depends on several factors.
- The severity of the meningioma and the treatment required.
- Whether the plaintiff needed surgery or other invasive treatments.
- The condition's impact on the plaintiff’s ability to work and overall quality of life.
- The strength of the scientific evidence linking Depo-Provera to the plaintiff’s condition.
Each case is unique, and an experienced attorney can help victims understand what compensation they may be entitled to based on their specific circumstances.
Talk to a Depo-Provera Attorney Today
The Lawsuit Legal News team is supported by Dolman Law Group, a law firm with extensive experience taking on big pharmaceutical companies in dangerous drug cases. We will help you pursue the compensation you deserve if Depo-Provera has harmed your health. Our team has successfully fought against major drug companies, including 3M, DuPont, Tylenol, and Pfizer, and we are prepared to take on the legal battle over Depo-Provera.
If you have been diagnosed with a meningioma or other serious health complications after using Depo-Provera, we want to hear from you. Our team is committed to holding pharmaceutical companies accountable for the harm caused by dangerous drugs and ensuring that victims receive the justice they are entitled to.
Our experienced Depo-Provera lawyers are ready to assist you with your claim and prepare for the inevitable filing of a lawsuit against Pfizer. We understand the physical, emotional, and financial toll that comes with dealing with a brain tumor or long-term health complications, and we are here to support you every step of the way.
We have built a national reputation for litigating against some of the largest pharmaceutical companies in the world. The lawyers of Dolman Law Group, who are affiliated with Lawsuit Legal News, have the experience and resources necessary to take on drug manufacturers nationwide.
We offer a free consultation and case evaluation to anyone who has experienced health problems linked to Depo-Provera. If you believe you have a potential claim, contact us today for a free consultation.
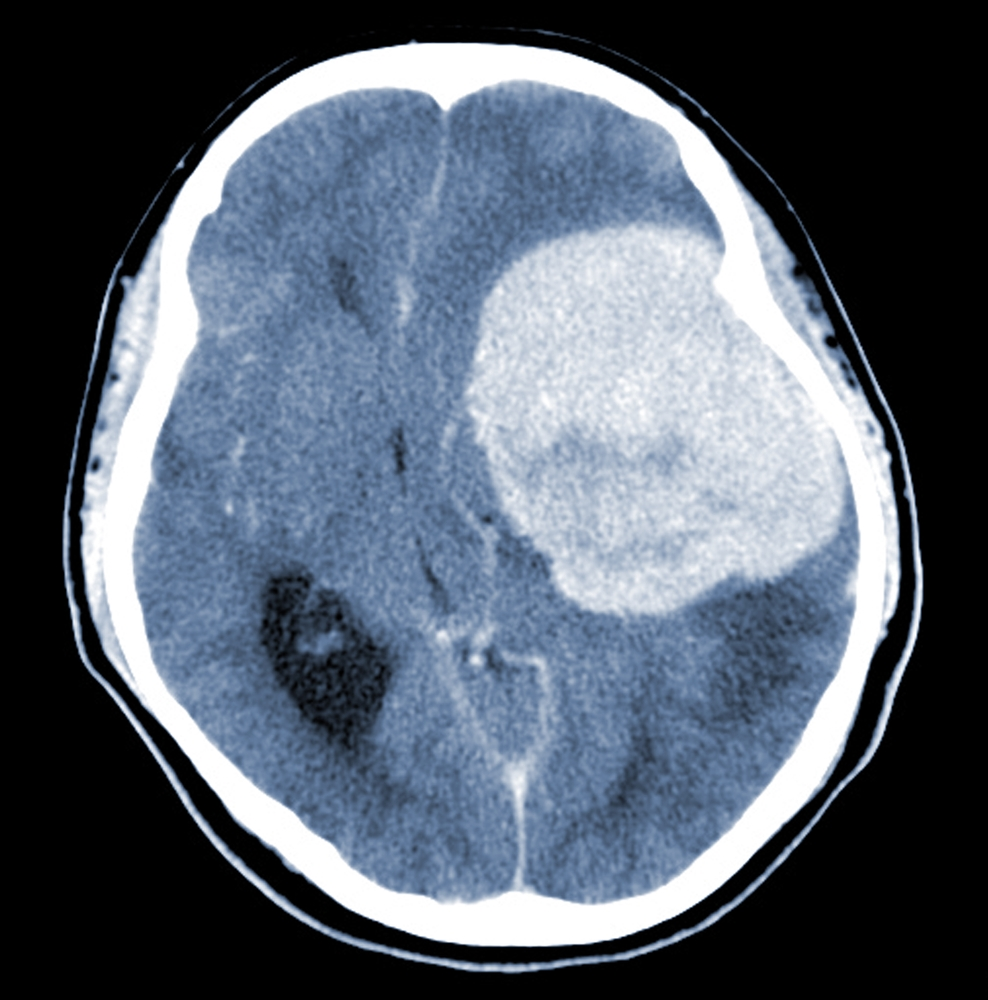
Real Stories from Women Who Have Suffered with Brain Tumors After Taking Depo-Provera
Middle-Aged Nurse Develops Brain Tumor—A 43-year-old nurse began experiencing severe headaches and difficulty understanding speech during work meetings. Initially attributing these symptoms to stress and fatigue, she sought medical attention when the issues persisted.
Diagnostic imaging revealed a meningioma—a type of brain tumor. Reflecting on her medical history, she noted prolonged use of hormone-based medications, including the Depo-Provera contraceptive injection, IVF treatments, and hormone replacement therapy (HRT), all containing progesterone.
Studies have linked certain progesterone-based medications to an increased risk of meningiomas, which are more prevalent in women. She underwent surgery to remove the tumor and is now recovering, though she continues to face memory challenges.
She advises women who have undergone extensive hormone treatments or experience persistent, unusual headaches to seek medical evaluation, including eye tests, as part of a proactive approach to their health.
Woman Suffers Golf Ball-sized Tumor—Another individual, a woman in her early 40s, experienced severe headaches and struggled with language comprehension, particularly during work meetings. These symptoms led to the discovery of a golf ball-sized brain tumor.
She attributed the tumor's growth to hormone treatments she received, including the Depo-Provera contraceptive injection, IVF, and HRT medication. Meningiomas, the type of tumor she had, are commonly non-cancerous and more frequent in females.
Studies have shown a link between hormone treatments, such as HRT and progesterone medications, and an increased risk of meningioma. Her symptoms began in December 2023, and she sought medical advice after a particularly disabling headache. A CT scan and MRI confirmed the brain tumor, leading to surgery in May 2024 to remove it. Post-surgery, she is recovering but advises other women who have undergone hormone treatments to get checked if they experience unusual headaches.