Suboxone, a medication for dealing with opioid addiction and treating withdrawal symptoms, can be a lifesaver. It helps by stopping cravings and making it difficult to get high on other dangerous drugs while taking it.
But lately, there has been a worrying discovery—a link between Suboxone sublingual strips and serious dental issues, such as the risk of severe tooth decay. Remember that only the oral film version of Suboxone causes tooth decay.
If you or someone you care about has had dental issues, such as tooth loss or gum injuries, while on prescription Suboxone film, we might be able to assist with obtaining compensation.
The Suboxone lawsuit lawyers at Dolman Law Group support Lawsuit Legal News, and we are here to help you determine your rights and what steps you can take to cover your medical bills and rebuild your life.
Multidistrict Litigation (MDL) Details
- MDL No. 3092 is centralized in the Northern District of Ohio before Judge J. Philip Calabrese
- As of mid-2025, approximately 890–900 cases are pending in the federal MDL
- Allegations center on the sublingual film version of Suboxone, which plaintiffs claim is acidic and causes tooth decay, enamel erosion, cavities, gum disease, and tooth loss
- Manufacturers are accused of failing to warn about these risks for over a decade
Cases We’re Currently Accepting
- Individuals who used Suboxone film or generic buprenorphine/naloxone strips
- Dental injuries, including:
- Tooth decay and enamel erosion
- Extractions, crowns, root canals
- Abscesses, infections, or gum disease
- Partial or complete tooth loss
Recent Key Litigation Developments
- June 2022 FDA label update acknowledged the risk of dental damage, but plaintiffs argue this change came too late for many users
- February 2024: JPML created MDL 3092 to consolidate Suboxone dental injury cases
- Mid-2024: Initial discovery phase begins; plaintiff leadership structure approved
- May 2025: Court allows “block filing” of hundreds of claims; bellwether case pool narrowed
- Summer 2025: Discovery continues, including document production and depositions
The Parties’ Litigation Positions
- Plaintiffs: Argue the formulation was defective due to its acidity, and that manufacturers failed to provide adequate warnings despite knowing of the risks
- Defendants: Claim risks were disclosed after the FDA label change, and that liability is limited by regulatory compliance; also argue that some claims lack jurisdiction or proof of causation
Major Concerns / Expectations
- Bellwether trials expected to begin in late 2025 or early 2026 — outcomes will guide future settlements
- Strong documentation (dental records, treatment timeline, Suboxone use history) will be critical to proving claims
- Legal complexity includes questions of federal preemption and whether manufacturers acted reasonably after the FDA warning
- No MDL resolution yet — parties are preparing for a multi-year litigation timeline unless early bellwethers shift momentum
Contact us online or call us at 866-535-9515 to decide whether or not you qualify to file a lawsuit.
Recent Suboxone Update—December 2025
We expect this to be a fast-moving case. The science linking sublingual Suboxone to tooth loss and other dental injuries appears strong. Of course, we will have to wait until both sides present their case, either to a jury or through mediation, but as it stands, we are confident in the claims made against Indivior.
Where can I get the latest update on the Suboxone lawsuit?
This page is an excellent resource for anyone looking to keep up with the latest news in the Suboxone lawsuit. We will provide easy-to-understand summaries of any court decisions, updates on any progress or changes in the case, and information about the potential settlement of the Suboxone case. We will also report on any new dental injuries that may arise in this case.
As Suboxone tooth decay lawsuits continue to be filed and the consolidated federal case against Suboxone (known as an MDL) progresses, the team at Lawsuit Legal News will provide regular and timely updates to keep you informed.
Our goal is to provide the most accurate and up-to-date information on all sites on the internet. If you have suffered severe tooth loss and decay while taking Suboxone sublingual strips, we want this page to be your go-to resource for new information.
Here are the latest updates on the lawsuit:
December 1, 2025 - Slight Decline in Suboxone Lawsuit Count
As of early November 2025, the number of Suboxone tooth damage lawsuits filed in federal court stands at 1,871, down slightly from the 1,877 reported last month. The small decrease reflects a mix of new filings and recent dismissals as the multidistrict litigation (MDL) continues to evolve.
Judge Allows Plaintiff to Proceed Despite Missed Deadlines
In a recent ruling, the MDL judge allowed a Suboxone plaintiff to move forward after initially missing court deadlines. The plaintiff, Michelle Robinson, was incarcerated for much of 2025 and unable to complete her required submissions on time. After her release in October, she submitted the necessary documentation. The court found she had shown good cause for the delay and denied the motion to dismiss her case.
This decision shows that while the court expects timely compliance, it is also willing to consider individual circumstances when legitimate reasons are provided.
Stay Informed About the Suboxone Litigation
Lawsuit Legal News continues to monitor the Suboxone litigation closely. With bellwether trials on the horizon and more than 11,000 plaintiffs involved behind the scenes, each update helps clarify the path ahead.
If you have questions about Suboxone-related tooth damage or want to stay informed about the legal process, the LLN team is here to help. Visit us often for the latest litigation developments.
November 1, 2025 - Judge Tightens Rules as Litigation Grows
Last month, the federal judge overseeing the Suboxone tooth decay multidistrict litigation (MDL) issued new orders aimed at streamlining the rapidly expanding case load and improving transparency from drugmakers Indivior and Aquestive. It’s estimated that more than 11,000 plaintiffs allege that the dissolvable Suboxone film caused severe dental injuries, including tooth decay and loss. While the docket officially lists fewer than 2,000 cases, many include dozens of plaintiffs filed in batches.
New Orders Target Recordkeeping and Manufacturer Disclosures
Judge Philip Calabrese now requires all plaintiffs who file after October 1, 2025, to submit census forms within 60 days. Older cases must meet a June 1, 2026, deadline. These forms are critical for tracking plaintiff data and moving viable cases forward.
In addition, the court ordered Indivior and Aquestive to produce key internal records, including FDA filings, marketing materials, and reports of dental injuries, to help determine what the companies knew about Suboxone’s risks.
Stan Gipe’s Role on the Plaintiffs’ Steering Committee
Attorney Stan Gipe of Dolman Law Group was appointed to the Plaintiffs’ Steering Committee to help guide the national litigation strategy. His work includes managing discovery issues, overseeing document production, and helping identify strong bellwether cases. Dolman Law Group created Lawsuit Legal News to provide updated information on pending mass tort litigation.
What to Expect Next
With bellwether trials approaching, pressure is building. A global Suboxone settlement isn’t expected until 2026, but these recent developments show the court is working to clear delays and force accountability. Lawsuit Legal News will continue to report as the litigation progresses, but if you have any questions, reach out for more information.
October 1, 2025 - Enforcement Ramps Up, Case Count May Be Nearing 20,000
Federal Judge Cracks Down on Delays
The Suboxone tooth decay litigation entered a more aggressive phase this month as Judge J. Philip Calabrese signaled he’s done tolerating missed deadlines and ignored court orders. During the September 9 status conference, the judge addressed procedural slowdowns and issued new warnings to both plaintiffs and third-party providers.
Three outside providers who failed to comply with record subpoenas will now be required to turn over documents or risk Rule 45 contempt proceedings, which can include court sanctions. Separately, the court dismissed with prejudice a number of plaintiff cases due to noncompliance with prior court orders. These dismissals are permanent, and the cases cannot be refiled.
While these steps may sound harsh, they are expected to benefit compliant plaintiffs by clearing out stale or unsupported claims and keeping the MDL moving toward bellwether trials and eventual settlement.
Plaintiff Census and Record Collection Update
The judge also announced that Census Round 2 will begin soon. These census forms help identify viable claims by collecting essential treatment and prescription information. Plaintiffs who fail to submit the required information risk losing their claims.
True Suboxone Case Count Might Top 20,000
Although the official MDL docket shows 1,882 filings, the actual number of individual plaintiffs is far higher due to batch-filing rules. Under a prior order, lawyers can include up to 100 plaintiffs per complaint. Based on current estimates, there may now be over 20,000 individual claims in the MDL—though not all will qualify for compensation.
Settlement Timeline Still Points to 2026
Despite some public speculation about an early resolution, most legal analysts continue to believe that a Suboxone settlement won’t happen until sometime in 2026. That’s when bellwether trials are expected to apply real pressure on Indivior, the drug’s manufacturer, to settle rather than face juries who will hear detailed evidence of the drug’s alleged dental harm.
September 2, 2025 - Timeline and Schedule Update for Suboxone Tooth Damage Lawsuit
The timeline of the multidistrict litigation for the Suboxone tooth decay lawsuits has changed a bit since we last discussed it, so we wanted to provide you with an update on what you can expect regarding upcoming proceedings.
Here’s where things stand now:
As we discussed below, Judge Calabrese held a ‘show‑cause hearing’ to find out why certain pharmacies have not complied with an earlier order to provide records to the court.
These records are vital for moving forward to the Bellwether Trials since they show who was prescribed Suboxone, when they began taking the sublingual films, and how long they were on the medication. Without these records, the litigation can’t move forward effectively. If Walgreens does not get the records together soon, they could be sanctioned by the court for their delay.
Bellwether Trials: The handful of trials that will act as gauges for both sides, known as bellwether trials, are now projected to begin in late 2025 or early 2026. These test cases play a huge, make‑or‑break role in the overall MDL settlement negotiations since they will give both sides valuable information about how juries are responding to the details of the Suboxone lawsuits.
Discovery Continues: At the current moment, although not much is happening in the public eye, there are still lots of things going on behind the scenes. Indivior is working on gathering its internal documents and other proof that it did not act negligently, while the plaintiffs are preparing depositions that will primarily focus on Suboxone’s 2022 label change, their marketing practices, and what they knew about the oral health concerns, when they knew it, and why they waited so long to add warnings to their products.
What’s Next In Suboxone MDL?
September 9, 2025: On Sep 9, an in-person hearing will be held to update the court and address any case management issues.
The court will also hold a status conference on this day for the Suboxone MDL, which we haven't had in a while. At this conference, both sides will update the court on their progress, address any procedural matters, and discuss the next steps, such as discovery updates and potential bellwether trials.
September 29, 2025: As September closes, the Court’s deadline for both sides to submit their fact sheets arrives. These fact sheets outline each side’s arguments.
For the plaintiffs, the fact sheets will include legal documents that summarize each plaintiff’s history with Suboxone, their dental injuries, and other relevant medical history. The defendant’s fact sheets will include their responses and defenses to the plaintiffs’ claims.
These fact sheets help both the court and the attorneys identify patterns across cases, which is essential for deciding which cases will be selected for the bellwether trials.
September 1, 2025 - Court Questions Pharmacies for Not Providing Records On Time, Increase in Batch Filings
On August 26, 2025, Judge Calabrese held a short but pointed show-cause hearing addressing four medical providers who failed to comply with previous court orders to turn over Suboxone-related records. Plaintiffs’ attorneys described months of outreach, including certified mail and repeated phone calls, without receiving the required documentation.
No representatives from the noncompliant providers appeared at the hearing. The court took the matter under advisement and directed the Clerk to send a copy of the hearing minutes to each entity. Plaintiffs also requested that the court impose monetary sanctions and require reimbursement of the legal costs incurred during the pursuit of these records.
Without pharmacy and provider records, plaintiffs cannot prove that they were prescribed Suboxone film, an essential element of these dental injury claims. The court’s willingness to consider sanctions may help break the impasse and push the litigation toward trial readiness.
Suboxone Filings Gain Traction in Batches
A new joint complaint filed in the MDL includes 54 plaintiffs from across the country. Each alleges severe dental injuries, such as tooth decay, erosion, and extractions caused by long-term use of Suboxone film, prescribed either for opioid use disorder or pain management.
The lawsuit claims the drug’s manufacturers failed to warn of Suboxone’s acidic formulation and its connection to irreversible dental damage. Plaintiffs say these risks were known to the company and supported by internal reports and scientific publications, but warnings were not added until June 2022, and only because of an FDA mandate.
If you were prescribed Suboxone and suffered serious dental problems, you may be eligible to join this litigation. The Lawsuit Legal News team can answer any questions and explain your rights during a free consultation.
August 1, 2025 - Status Conference Yields Key Progress, Invidior May Be Misjudging Public Opinion
Recently, the MDL judge held a two-hour Zoom status conference to track the progress of the Suboxone tooth decay litigation. The result? Some key agreements and deadlines show that this case is moving forward.
Agreements reached:
- How to handle missing documents from plaintiffs
- Redacting older deposition transcripts
- Finalizing authorization forms for treatment records
- Tracking claims filed after October 7, 2024
- Managing future document production from relevant individuals
Upcoming deadlines:
- August 15, 2025 – Propose a plan for collecting documents from non-custodial sources or identify any disagreements
- September 29, 2025 – Submit fact sheets summarizing each side’s claims and defenses
The next in-person hearing is scheduled for September 9, 2025.
Deposition Procedures Finalized
On July 8, a new order outlined the rules for all upcoming depositions in the Suboxone MDL. Highlights include:
- In-person depositions are the default; Zoom is allowed if both sides agree
- Remote depositions must be secure and private
- Exhibits must be shared with witnesses ahead of time when possible
- Defense must offer two available dates within 10 days when plaintiffs request to depose company personnel
- Each side gets one lead examiner per deposition; plaintiffs can split questioning between two attorneys with advance notice
Settlement Strategy: Indivior’s Risky Gamble
Indivior seems to believe juries won’t sympathize with Suboxone users in recovery. That assumption may backfire—badly.
- These plaintiffs are teachers, nurses, warehouse workers, single parents, basically regular people who followed their doctors’ treatment plans and trusted Suboxone.
- Instead, they ended up with severe dental injuries, including broken teeth, chronic pain, and in some cases, complete dentures by age 40.
- Jurors won’t ignore that. If Indivior thinks stigma will protect them in court, they’re misreading the cultural moment—which could cost them billions.
Suboxone Cases Are Gaining Visibility
SuboxSuboxone lawsuits have flown under the radar—until now. The tide is turning.
- As more people in recovery speak out, the emotional weight of their stories is gaining traction.
- Many had no history of dental problems before taking Suboxone.
- Now they face thousands of dollars in unreimbursed dental care, lost confidence, and lasting pain, after receiving no warning from the drugmaker.
A single high-profile trial could make this litigation national news and put real pressure on Indivior to settle.
July 1, 2025 - Records Delays, Plaintiff Changes, and Settlement Timeline Insights
As the Suboxone tooth decay litigation gains momentum, several key developments are shaping the road ahead for victims seeking justice. From pharmacy records delays to updates on trial preparations and the timeline for potential settlements, here’s what’s happening now—and what it means for you.
Pharmacy Record Access Still a Hurdle
Obtaining pharmacy records continues to be a frustrating roadblock for many plaintiffs. These records are essential for proving Suboxone use, but not all pharmacies are cooperating. Last month, the court issued a show-cause order against Walgreens, Safeway, and Porch Light Health for allegedly failing to produce records within 30 days, as required by Case Management Order No. 13.
This week, the judge dropped the order for Safeway and Porch Light after confirming their compliance, but Walgreens remains on the hook. A hearing is set to determine whether the retail giant has been meeting its legal obligations. For plaintiffs trying to move their cases forward, these delays are more than a nuisance; they can hold up everything..
Plaintiff Pool Refreshed in Trial Prep Process
Recently, 48 plaintiffs were removed from the initial 500-person Records Collection Pool due to voluntary dismissals or failure to submit required documentation. These individuals have now been replaced with new plaintiffs, randomly selected according to the established trial protocol.
This is important because these 500 cases form the backbone of the bellwether trial (test trials) selection process. From this pool, 100 will move to deeper discovery, and eventually, four key cases will go to trial. This ongoing refinement keeps the MDL on track and ensures that well-documented, trial-ready cases rise to the top.
We Don’t Expect a Suboxone Settlement Just Yet
If you’re wondering when settlement payouts might begin, the short answer is... no time soon. While there’s always buzz about early deals, the more realistic expectation is that meaningful settlement talks won’t pick up steam until late 2025 or even early next year.
Why the delay? Defense attorneys for Indivior, the maker of Suboxone, are likely waiting for the three-year statute of limitations to expire, which should be around now. Settling too early could trigger a wave of new lawsuits, which the company would rather avoid. Waiting allows them to limit risk and control the number of claims. To learn whether you still have a viable Suboxone claim, reach out to the LLN team today.
June 1, 2025 - Suboxone Litigation Moves Toward First Trials as Case Count Nears 900
Plaintiffs across the country allege that Suboxone film, a medication used in opioid addiction recovery, caused severe tooth decay and dental injuries, and that the manufacturer, Indivior, failed to adequately warn of this risk.
New Case Management Order Sets Bellwether Trial Process
On May 30, the MDL judge issued Amended Case Management Order No. 15, outlining a detailed structure for selecting bellwether trial cases. The order creates a 500-member Records Collection Pool, from which plaintiffs must submit medical authorizations to verify Suboxone treatment and related dental harm. Noncompliant cases may be dismissed and must be promptly replaced to maintain the pool size.
From this group, 100 cases will be randomly selected to move into the Core Discovery Pool, where both sides will exchange fact sheets and medical documentation through the secure Rubris Crosslink platform. That group will be narrowed to 50 cases, balanced between randomly chosen, plaintiff-selected, and defense-selected plaintiffs.
After discovery and depositions, 15 cases will form the Trial Pool—five selected by each side and five randomly chosen. Finally, six potential trial cases will be named, with each party striking one to finalize four bellwether trials. All trials will proceed as single-plaintiff cases.
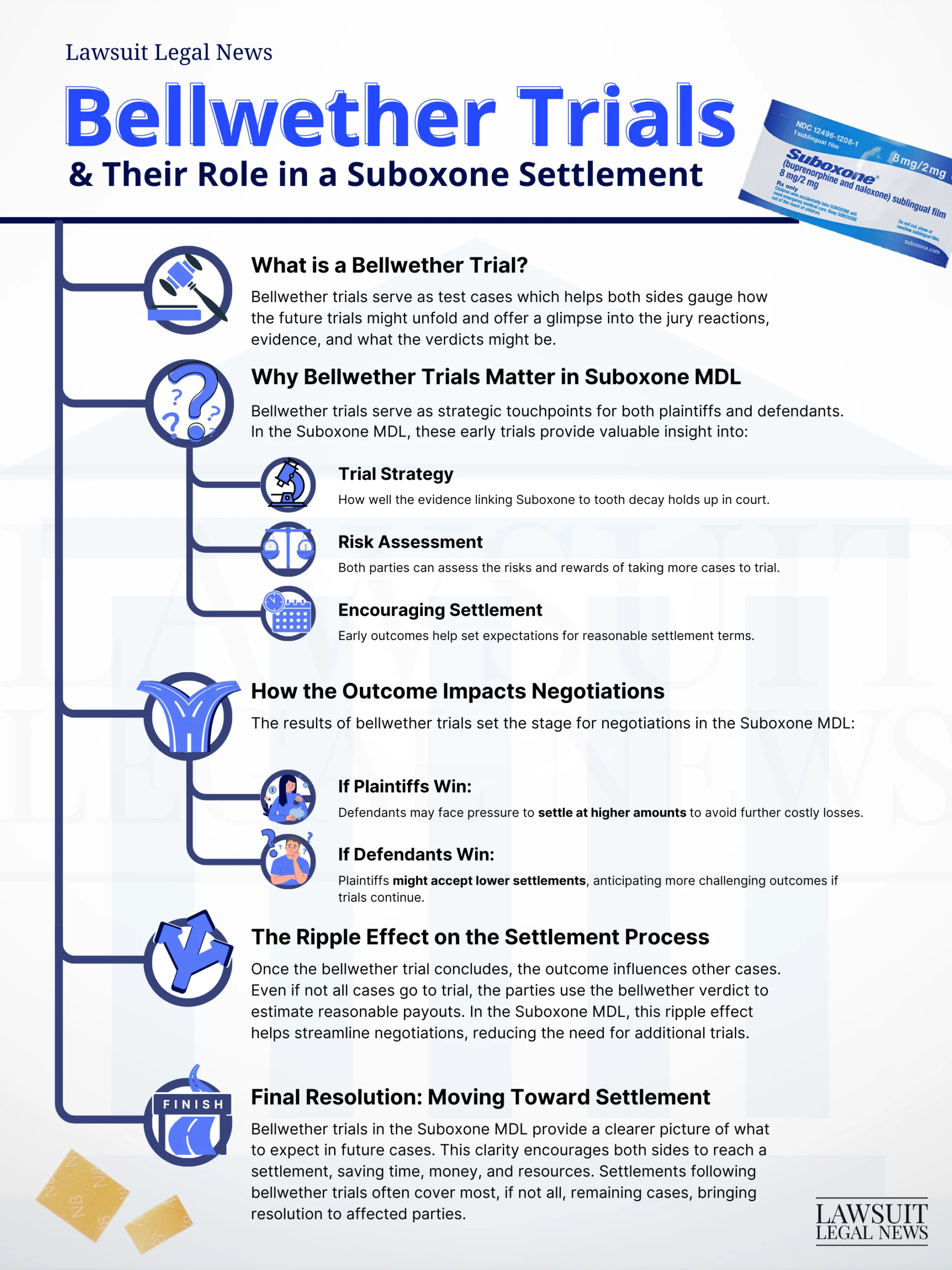
The Stakes for Indivior
As this litigation progresses, legal observers warn that Indivior may be miscalculating its exposure. The company appears to be betting that jurors will lack sympathy for individuals recovering from addiction. But plaintiffs’ attorneys strongly disagree. They argue jurors will see past outdated stigmas and focus on the fact that these are real people—parents, workers, and spouses—who got clean and trusted Suboxone to help them stay there.
Instead, many were blindsided by painful, costly dental injuries. From broken and rotting teeth to repeated dental surgeries and tens of thousands in out-of-pocket costs, these stories are powerful. Plaintiffs claim Indivior never warned about this risk, leaving patients vulnerable to permanent harm.
Should these cases proceed to trial, attorneys believe juries will empathize with victims and that any attempt to downplay their suffering could backfire significantly. A fair early settlement, they argue, would likely cost Indivior far less than letting this play out in front of sympathetic jurors.
Case Count Update: More Lawsuits on the Way
As of early May, the MDL had grown to 896 cases, up from 884 the previous month. But this modest increase doesn’t reflect what’s coming. Lawyers are now filing complaints in blocks of 100 plaintiffs per suit, meaning a large surge is expected in June.
May 1, 2025—Suboxone Dental Injury Lawsuits Status
Hundreds of individuals have filed lawsuits alleging that Suboxone, a medication used for opioid use disorder, caused severe dental damage, including tooth decay and tooth loss. These cases are now part of coordinated federal litigation in Ohio.
Overview of the Suboxone MDL
- MDL Number: 3092
- Location: U.S. District Court for the Northern District of Ohio
- Presiding Judge: J. Philip Calabrese
- Case Volume: As of May 1, 2025, approximately 896 Suboxone dental injury cases are consolidated in this MDL.
- Trial Status: No bellwether trials have been scheduled or held yet. Settlement discussions have not led to any approved resolutions at this time.
Recent Key Developments
- Mass Filing Streamlined:
- The court approved Case Management Order No. 14, allowing up to 100 plaintiffs to be included in a single complaint.
- This order was designed to reduce filing fees and manage the growing number of incoming cases more efficiently.
- Discovery Advancing Toward Bellwether Trials:
- By mid-April, attorneys on both sides had selected a group of 500 plaintiffs for targeted discovery.
- This process includes collecting dental records and treatment history to help identify cases for future bellwether trials.
- Discovery efforts are expected to inform the eventual selection of test cases later in 2025.
- Depositions and Corporate Accountability:
- Plaintiffs intend to take corporate depositions under Rule 30(b)(6) from Indivior, the manufacturer of Suboxone.
- Topics include:
- The company’s response to dental injury complaints from Suboxone users.
- Details surrounding the 2022 label update ordered by the FDA, which added a warning about dental risks.
- The structure of Indivior and any related corporate entities involved in Suboxone's development and distribution.
- Third-Party Discovery Enforcement:
- At a status conference held on April 17, 2025, the court addressed discovery delays.
- Judge Calabrese scheduled a show-cause hearing for CVS Pharmacy, which had failed to comply with a subpoena for patient records relevant to the litigation.
What Comes Next
- The litigation remains in the pre-trial discovery phase, with no trial dates set yet.
- Regular status conferences keep the case on track.
- Bellwether case selection is expected to begin once the discovery phase for the initial plaintiff pool is complete.
This MDL reflects growing legal scrutiny of the potential dental harm associated with Suboxone, particularly following the FDA's 2022 label change that acknowledged dental risks. More filings are expected as awareness of the litigation spreads.
Earlier Suboxone Litigation News

As of February 2024, the Suboxone Multidistrict Litigation (MDL) was created to consolidate all federal cases for pretrial procedures. The MDL is following the typical process for dangerous drug lawsuits. Both sides are holding their positions and asking the court to grant motions in their favor. Discovery continues while more evidence is gathered to support the link between Suboxone use and the serious dental problems many plaintiffs face.
New cases are joining the MDL each month as more Suboxone users are discovering the link between the drug and their injuries. We are currently accepting clients who have experienced at least three lost teeth from Suboxone use, either from a dental extraction or from falling out.
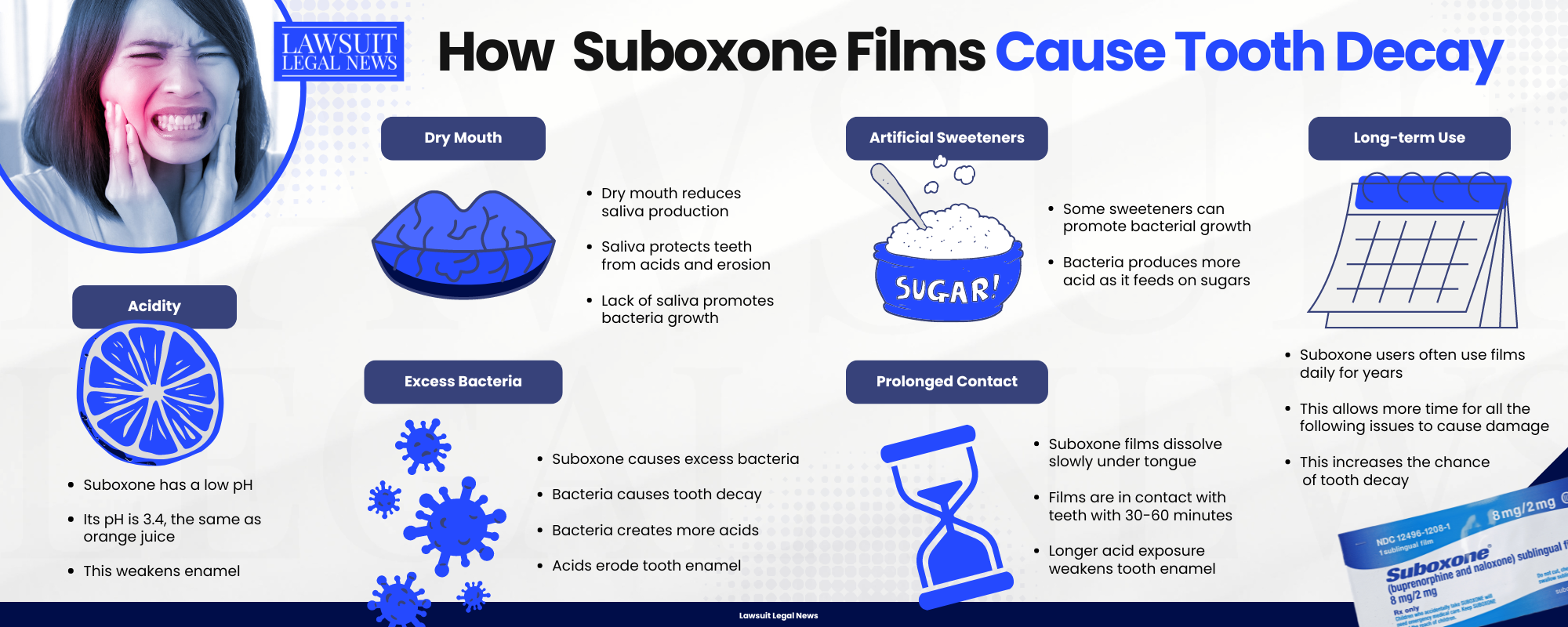
Frequently Asked Questions About the Suboxone Lawsuits
What is the link between Suboxone and tooth decay?
Suboxone sublingual film, used to treat opioid use disorder, has been linked to severe dental problems including tooth decay, gum disease, oral infections, and even complete tooth loss. The issue arises because the film is placed under the tongue and dissolves slowly, exposing teeth to acidic conditions for extended periods. This prolonged exposure can damage the enamel and lead to rapid dental deterioration. The dental risks are serious enough that the FDA issued a warning and required changes to the product’s label in 2022.
Does Suboxone always cause dental problems?
No, not all forms of Suboxone carry the same risk. The dental issues reported are specifically linked to the sublingual film version of Suboxone, which is placed under the tongue and dissolves directly in the mouth. The tablet version of Suboxone, which is swallowed rather than absorbed sublingually, does not appear to cause the same kind of dental damage and is not subject to the same FDA warning. Therefore, individuals using Suboxone tablets are generally not considered at risk for the same level of tooth decay.
When did Suboxone begin warning users about dental risks?
The FDA required a warning label update in June 2022 after receiving numerous reports of dental injuries caused by Suboxone sublingual film. These injuries included tooth decay, tooth fractures, infections, and in some cases, total tooth loss. The FDA noted that while these side effects were serious, they were preventable and should have been clearly communicated to both patients and healthcare providers. Unfortunately, before this change, there was no direct warning about dental risks on Suboxone’s label, which is a key point in the lawsuits being filed against the manufacturer.
Can I file a lawsuit for dental damage caused by Suboxone?
Yes. If you used Suboxone sublingual film and suffered from significant dental issues like cavities, extractions, oral infections, or tooth loss, you may be eligible to file a lawsuit against Indivior, the manufacturer of Suboxone. These lawsuits allege that Indivior failed to adequately warn users and healthcare providers about the risks associated with using the sublingual film, despite having access to reports and data that could have alerted them to the dangers. People who used the drug before the 2022 warning and experienced dental harm are especially likely to qualify for a claim.
What kind of dental injuries are linked to Suboxone film?
Users of Suboxone sublingual film have reported a wide range of serious dental injuries. These include rapidly progressing cavities, severe enamel erosion, gum damage, tooth fractures, painful oral infections, and full tooth loss. Many individuals required extensive dental work such as root canals, crowns, implants, and even full dentures. The damage often appeared suddenly in individuals with no prior history of significant dental issues, making the connection to Suboxone use even more apparent.
Is there ongoing litigation against Suboxone's manufacturer?
Yes, there is currently a growing number of lawsuits filed against Indivior over Suboxone-related dental injuries. As of 2025, approximately 900 lawsuits have been consolidated in a Multidistrict Litigation (MDL) in the Northern District of Ohio. These cases accuse Indivior of failing to provide adequate warnings and misleading consumers about the safety of the sublingual film. The MDL structure helps streamline the legal process for plaintiffs while the courts assess common questions related to the case.
What is the current status of the Suboxone lawsuits?
The Suboxone MDL is currently in the discovery and bellwether trial selection phase. The first bellwether trials are expected to begin in late 2025. These early trials serve as test cases to gauge how juries may respond to the evidence and can influence future settlement discussions. While no settlements have been announced yet, the litigation is progressing, and the outcome of the bellwether trials may pave the way for compensation for thousands of affected individuals.
What evidence do I need to file a Suboxone tooth decay claim?
To successfully file a claim, plaintiffs will need to provide documentation that supports both their use of Suboxone sublingual film and the resulting dental injuries. This typically includes:
- Pharmacy records or prescriptions proving Suboxone film usage
- Dental records showing the extent and nature of the tooth damage
- A timeline showing when the injuries occurred in relation to Suboxone use
- Any additional proof of dental treatments or out-of-pocket expenses
This evidence helps establish a link between the drug and the injury and is essential for building a strong legal case.
Why didn’t patients or doctors know about Suboxone’s dental risks sooner?
According to lawsuits, Indivior failed to adequately warn both patients and healthcare professionals about the risk of dental injury. Even when the label was eventually updated, the warning was buried within a long list of side effects and no direct communication (such as a “Dear Doctor” letter) was made. Many prescribers and users remained unaware of the risk until they experienced or observed the damage firsthand. Plaintiffs argue this lack of clear, timely communication constitutes negligence.
Are Suboxone lawsuits still being accepted?
Yes, law firms are still accepting new Suboxone dental injury cases. Whether a person is eligible depends in part on when they used Suboxone and the statute of limitations in their state. Many states have statutes that allow claims to be filed within 2–3 years from when the injury was discovered or reasonably should have been discovered. Because the FDA warning wasn’t issued until 2022, there may still be time for new plaintiffs to come forward in 2025.
What is Suboxone?
Suboxone is a medication—often prescribed in a film rather than a tablet, like the picture above—that aids individuals struggling with opioid addiction by making it easier for them to stop using the harmful drugs.
It works similarly to other opiates but in a way that doesn't make you feel "high." This can reduce cravings and withdrawal symptoms, making it easier to recover from opioid dependence or addiction. Attaching to the same parts of the brain that opioids stick to attaching to the same parts of the brain that opioids stick to
Suboxone is generally considered the best in class for opioid addiction treatment. Some individuals might get side effects from Suboxone, like nausea, headaches, and constipation. But if you notice anything concerning dental health, especially tooth decay, you should talk to a doctor.
Suboxone Tooth Decay Lawsuits
Suboxone tooth decay lawsuits involve claims against the manufacturers of Suboxone. Plaintiffs allege that the drug causes severe tooth decay and dental problems, which the manufacturer, Indivor, did not adequately disclose. The lawsuits argue that users experienced significant dental issues, such as complete tooth loss, because of the medication. These cases highlight the need for more explicit warnings and better information about the potential side effects of drugs.
It also asks, "What should be done when companies know about dangers but choose not to share the information to protect corporate profits?"
Why are People Filing Lawsuits
Dental health problems linked to Suboxone have become a problem for those prescribed the medication. Many have reported some severe dental issues, including:
Severe Tooth Decay
This is one of the biggest issues, resulting in numerous cavities and damaging teeth, often resulting in emotional distress. The majority of cases we have seen so far are potential tooth decay lawsuits.
Tooth Erosion
Suboxone use might erode tooth enamel, making teeth more vulnerable to decay and sensitivity. As a result, patients can develop the following dental problems:
- Cavities
- Dental caries
- Dental crowns or crown replacement
- Tooth loss
Dry Mouth
Some people on Suboxone experience dry mouth as a side effect, leading to less saliva production. Saliva helps protect teeth from decay, so less of it can mean more dental problems. Saliva also protects against dental caries (the breakdown of the tooth).
Gum Problems
Suboxone might also cause gum issues, like inflammation, which can significantly impact dental health, and periodontal disease, which also has a profound impact on dental health.
Tooth Fractures/Broken Teeth
We are seeing numerous complaints of fractured teeth and cracked teeth supposedly caused by Suboxone film strips.
Infections
Many of the aforementioned dental problems also come with the risk of leading to infection. Mouth and dental infections are painful and costly and can potentially spread. Depending on the person, infections can also present severe risks if they are immunocompromised or elderly. Tooth infections can also spread to other parts of your head and cause infections, abscesses, blood infections, and even heart and kidney damage.
Tooth Loss
Tooth loss due to decay or infection occurs when a tooth or multiple teeth become severely damaged by decay or advanced dental infections. As the decay or infection spreads, it weakens the tooth structure and surrounding tissues, eventually leading to tooth loss. This type of tooth loss can cause severe pain, problems eating and chewing, and many other issues. Prompt treatment can prevent such outcomes, including fillings, root canals, or extractions.
Fixing these dental problems can be painful and expensive.
How to Qualify for a Suboxone Lawsuit
- You must have used prescribed Suboxone sublingual strips for at least six months.
- You must have suffered a dental injury or severe dental health problems after starting Suboxone (including any of the following injuries: advanced tooth decay, tooth loss, tooth fracture, substantial cavities, gum disease, and gum injuries).
- You must have undergone routine dental care before using Suboxone, so you have a record of your prior dental health.
Allegations Against Indivior for Suboxone Tooth Decay
The lawsuits claim that Indivior didn't warn people enough about the risk that a user may suffer severe tooth decay linked to the opioid addiction treatment drug Suboxone. They also say that Indivior knew or should've known about the risk but didn't do enough to prevent it. Our Suboxone lawyers believe we will learn more over the coming months about how much Indivior knew about the risks of sublingual Suboxone film exposure years ago.
Evidence for Suboxone Causing Tooth Damage
The connection between prescription medication Suboxone use and tooth decay is based on reports and scientific observations. But we need more research to be sure. Here's what's been pointed out (potential side effects):
Evidence for Suboxone Causing Tooth Damage
The connection between the prescription medication Suboxone and tooth decay is based on reports and scientific observations. Although more research is needed to establish a definitive link, several potential side effects have been identified. Here’s a detailed look at the evidence:
Patient Reports
- Widespread Anecdotal Evidence: Numerous individuals taking Suboxone (the gold standard medication for treating opioid use disorder and curbing withdrawal symptoms) have reported significant dental issues, including tooth degradation, discoloration, and loss. These patient reports have been a primary driver in raising awareness and prompting further investigation into the dental side effects of Suboxone.
- Forums and Support Groups: Online forums and support groups for people recovering from opioid addiction often discuss dental problems associated with Suboxone use, indicating a pattern among users that warrants scientific scrutiny.
Dry Mouth Side Effect
- Saliva Production Reduction: Suboxone is administered sublingually (under the tongue), which can significantly reduce saliva production. Saliva is crucial for maintaining oral health as it helps neutralize acids produced by bacteria, wash away food particles, and provide disease-fighting substances.
- Consequences of Dry Mouth: The reduction in saliva leads to a condition known as xerostomia, which can increase the risk of tooth decay, gum disease, and oral infections. Patients often experience a higher incidence of cavities and other dental issues when their mouth remains dry for extended periods.
How Does Saliva Protect Your Teeth?
Saliva plays a crucial role in maintaining oral health and protecting teeth. It contains enzymes and proteins that help neutralize acids produced by bacteria, which can cause tooth decay. Saliva also washes away food particles and debris, reducing the risk of cavities.
It also provides essential minerals, such as calcium and phosphate, which help remineralize and strengthen tooth enamel. Saliva's lubricating properties facilitate easier swallowing and chewing, forming a protective coating on teeth, further shielding them from harmful substances. Overall, saliva is essential for preserving dental health and preventing oral diseases.
Acidic Nature
- pH Levels of Suboxone Strips: Suboxone strips have an acidic pH, typically around 3.5 to 4.5. This acidity can directly contribute to tooth enamel erosion, the protective outer layer of teeth.
- Enamel Erosion: Continuous exposure to acidic substances can weaken enamel, making teeth more susceptible to decay and sensitivity. Without diligent dental hygiene practices, the acidic nature of Suboxone can exacerbate these issues.
Medical Studies
- Preliminary Research Findings: Initial studies have suggested a correlation between the use of opioid medications like Suboxone and dental problems. These studies indicate that opioid use can negatively impact oral health, but more comprehensive research is necessary to confirm these findings and understand the mechanisms involved.
- Need for Further Research: Current research is limited and often anecdotal. Comprehensive, long-term studies are required to establish the connection between Suboxone and dental health issues, considering various factors such as dosage, duration of use, and individual patient differences.
While significant anecdotal evidence and some preliminary research suggest a link between Suboxone use and tooth damage, more rigorous scientific studies remain necessary. Suboxone patients should be aware of these potential side effects and take proactive measures to maintain oral health, including regular dental check-ups and proper dental hygiene practices.
Possible Punitive Damages
We believe Indivior knew just how acidic the sublingual strips of Suboxone are and how they can destroy a user's tooth enamel. Numerous adverse event reports have been received, including severe tooth decay, broken teeth, cracked teeth, and gum infections, among other issues related to the use of the Suboxone sublingual form.
Indivior's failure to warn consumers of such issues has resulted in the need for extensive dental treatment for many of the clients we represent. Thus, we believe punitive damages are warranted above and beyond compensatory damages in a Suboxone dental lawsuit.
Studies Connecting Suboxone to Tooth Decay
A few studies have looked into the connection between Suboxone and tooth decay. Some credible sources suggest that Suboxone might increase the risk of dental problems:
- One study in 2016 found that people taking Suboxone were more likely to get tooth decay than those who didn't.
- Another study found that Suboxone users had more cavities and tooth erosion than others.
- A 2022 study found that Suboxone users had a higher risk of dental issues compared to those taking other meds for opioid use disorder, especially if they had dental problems before starting Suboxone. There was a large increase in the risk for adverse dental outcomes.
Potential Injuries in a Suboxone Case
- Tooth Enamel Loss
- Cavities
- Cracked Teeth
- Tooth Decay
- Infections
- Tooth Loss and Extraction
- Total Tooth Loss
FDA's Response to Suboxone Tooth Decay
In 2022, the FDA warned about the risk of dental problems linked to buprenorphine (Suboxone). Some people had severe oral issues like tooth decay, cavities, infections, and tooth loss, even if they'd never had dental problems before and maintained good dental hygiene.
According to the FDA, increasing medical research and reports connect the dissolvable sublingual film with dental problems. So, the FDA told manufacturers to include warnings about these dental risks in the prescribing information and the patient medication guide.
We believe the drug maker Indivior, Inc. failed to include adequate warnings the sublingual film version of this prescription drug could cause serious dental health issues.
Statute of Limitations for the Suboxone Dental Decay Lawsuit
July 7, 2024 update - We are now taking cases in every state and will argue that the warning in the insert for Suboxone film is hardly a warning. Further, Indivior has never provided a "Dear Doctor" letter to physicians to prescribe Suboxone film, which would have placed such medical professionals on notice of the potential dental injuries related to the medication.
The statute of limitations in mass tort cases is one of those things that is always a concern. For example, lots of people took the medication years ago and are just now finding out about all the damage it caused. Or, maybe five years ago, they suffered severe dental problems, but they didn't know it was because of Suboxone films.
The statute of limitations for the Suboxone Tooth Decay Lawsuit depends on the state in which you live and the specific circumstances of your case.
Specifically, it depends on the window of opportunity established by the statute of limitations for defective drug cases or product liability cases in your state. And whether or not your specific circumstances will allow a lawyer to still file a lawsuit on your behalf, even if the deadline may have passed. For example, if your dental injuries just started showing, a lawyer may be able to work with you.
It should be noted that in a lot of states, the statute of limitations may have run out since Indivior added a warning to Suboxone film in January 2022.
Calling the LLN Suboxone Lawyers is the quickest way to find out, but there is a breakdown of each state and its statute of limitations pertaining to defective drugs or product liability. Those states in bold have reached or are very near the end of their statute of limitations. Please refer to this list if you are considering filing a Suboxone lawsuit.
- Alabama Statute of Limitations for Defective Drug Case: 2 Years
- Alaska Statute of Limitations for Defective Drug Case: 2 Years
- Arizona Statute of Limitations for Defective Drug Case: 2 Years
- Arkansas Statute of Limitations for Defective Drug Case: 3 Years
- California Statute of Limitations for Defective Drug Case: 2 Years
- Colorado Statute of Limitations for Defective Drug Case: 2 Years
- Connecticut Statute of Limitations for Defective Drug Case: 2 Years
- Washington D.C. Statute of Limitations for Product Liability: 3 Years
- Florida Statute of Limitations for Product Liability: 4 Years
- Delaware Statute of Limitations for Product Liability: 2 Years
- Georgia Statute of Limitations for Defective Drug Case: 2 Years
- Hawaii Statute of Limitations for Product Liability: 2 Years with discovery rule
- Idaho Statute of Limitations for Product Liability: 2 Years
- Illinois Statute of Limitations for Product Liability: 2 Years
- Indiana Statute of Limitations for Product Liability: 2 Years
- Iowa Statute of Limitations for Product Liability: 2 Years
- Kansas Statute of Limitations for Product Liability: 2 Years
- Kentucky Statute of Limitations for Product Liability: 1 Year with discovery rule
- Louisiana Statute of Limitations for Product Liability: 1 Year
- Maine Statute of Limitations for Product Liability: 6 Years
- Maryland Statute of Limitations for Product Liability: 3 Years
- Massachusetts Statute of Limitations for Product Liability: 3 Years
- Michigan Statute of Limitations for Product Liability: 3 Years
- Minnesota Statute of Limitations for Defective Drug Case: 6 Years
- Mississippi Statute of Limitations for Product Liability: 2 Years
- Missouri Statute of Limitations for Product Liability: 5 Years
- Montana Statute of Limitations for Product Liability: 3 Years
- Nebraska Statute of Limitations for Product Liability: 2 Years
- Nevada Statute of Limitations for Product Liability: 2 Years
- New Hampshire Statute of Limitations for Product Liability: 3 Years
- New Jersey Statute of Limitations for Product Liability: 2 Years
- New Mexico Statute of Limitations for Product Liability: 3 Years
- New York Statute of Limitations for Product Liability: 3 Years
- North Carolina Statute of Limitations for Product Liability: 3 Years
- North Dakota Statute of Limitations for Product Liability: 10 Years
- Ohio Statute of Limitations for Product Liability: 2 Years
- Oklahoma Statute of Limitations for Product Liability: 2 Years
- Oregon Statute of Limitations for Product Liability: 2 Years
- Pennsylvania Statute of Limitations for Product Liability: 2 Years
- Rhode Island Statute of Limitations for Product Liability: 3 Years
- South Carolina Statute of Limitations for Product Liability: 3 Years
- South Dakota Statute of Limitations for Product Liability: 3 Years
- Tennessee Statute of Limitations for Product Liability: 1 Year with discovery rule
- Texas Statute of Limitations for Product Liability: 2 Years
- Utah Statute of Limitations for Product Liability: 2 Years
- Vermont Statute of Limitations for Product Liability: 3 Years
- Virginia Statute of Limitations for Product Liability: 2 Years
- Washington Statute of Limitations for Product Liability: 3 Years
- West Virginia Statute of Limitations for Product Liability: 2 Years
- Wisconsin Statute of Limitations for Product Liability: 3 Years
- Wyoming Statute of Limitations for Product Liability: 4 Years
Compensation for Suboxone-Related Tooth Decay
People who've had severe tooth decay or other mouth problems because of Suboxone might be able to get some compensation. The amount depends on the extent of the dental damage and the case details.
Compensation for tooth problems related to Suboxone film use could include:
- Medical Bills
- Dental Bills
- Pain and Suffering
- Lost Wages
- Future Medical Costs
- Emotional distress
- Punitive damages
Read more: How much can I get in a Suboxone settlement?
Indivior's Questionable Past as a Bad Actor
Indivior agreed to a $385 million settlement in October to resolve a series of lawsuits that accused it of illegally attempting to maintain its monopoly over Suboxone, a medication used to treat opioid addiction.
In June, Indivior agreed to pay a settlement of $102.5 million to dozens of U.S. states for the monetary damage they incurred through state-based healthcare programs. And, in August, they also agreed to pay $30 million to settle a similar class action lawsuit by health insurance companies.
These lawsuits show that Indivior is no stranger to serious accusations.
More to the point, this lawsuit and others like it filed against Indivior also bolster claims that Suboxone strips did not have a warning about tooth decay because the product was rushed to market to help maintain the monopoly addressed in this lawsuit, presumably.
The lawsuits allege that Indivior attempted to extend its monopoly by changing Suboxone from a tablet form to a sublingual film version to deter generic competition by convincing doctors and patients that new—and still Indivior-owned—films were more effective and convenient for users, making them a better choice than the tablets.
This was never proven to be the case, but today, the sublingual film version of Suboxone completely dominates the market, even now that both tablet and film patents are up. Their marketing worked: strips have dominated tablets in Suboxone treatment.
Indivior settled without admitting any wrongdoing and concluded nearly ten years of constant litigation.
That is, until it was discovered that they failed to warn patients that Suboxone can cause possible tooth decay, whether or not it was caused by rushing to maintain their monopoly.
Talk To a Suboxone Lawyer Today
Lawsuit Legal News is ready to help you get the compensation you need for your dental problem caused by Suboxone. Our associated law firm, Dolman Law Group, has fought back against some of the biggest drug companies in the world, like 3M, Chemguard, Chevron, Tyco, Dupont, Tylenol, and Ozempic, and we are ready to take on Indivior.
If you suffer from or have suffered severe tooth decay, tooth loss, fractured teeth, root canal, or persistent cavities after using the sublingual form of Suboxone film, we would like to speak with you.
Our Suboxone lawyers remain ready, willing, and able to assist you with handling your claim and the inevitable filing of a lawsuit against Indivior. Dealing with opioid dependence is a significant ordeal by itself. Having to tackle an opioid use disorder and withdrawal symptoms while dealing with poor dental health is quite a burden.
We have built a national reputation for litigating against every major pharmaceutical company that has injured patients taking their drugs. The Dolman lawyers affiliated with Lawsuit Legal News have taken on drug manufacturers nationwide.
Our Suboxone tooth decay lawyers offer a free consultation and case evaluation to anyone suffering from tooth loss, severe tooth decay, tooth erosion, gum disease, and other dental injuries or tooth issues following long-term use of Suboxone. Contact us for a free consultation if you have a potential lawsuit.We are handling Suboxone tooth decay cases nationwide. Contact us immediately at (833) 552-7274.
- The infographic is the sole property of Lawsuit Legal News and cannot be used, copied, or downloaded without LLN's consent. You may, however, link to the infographic. ↩︎